1
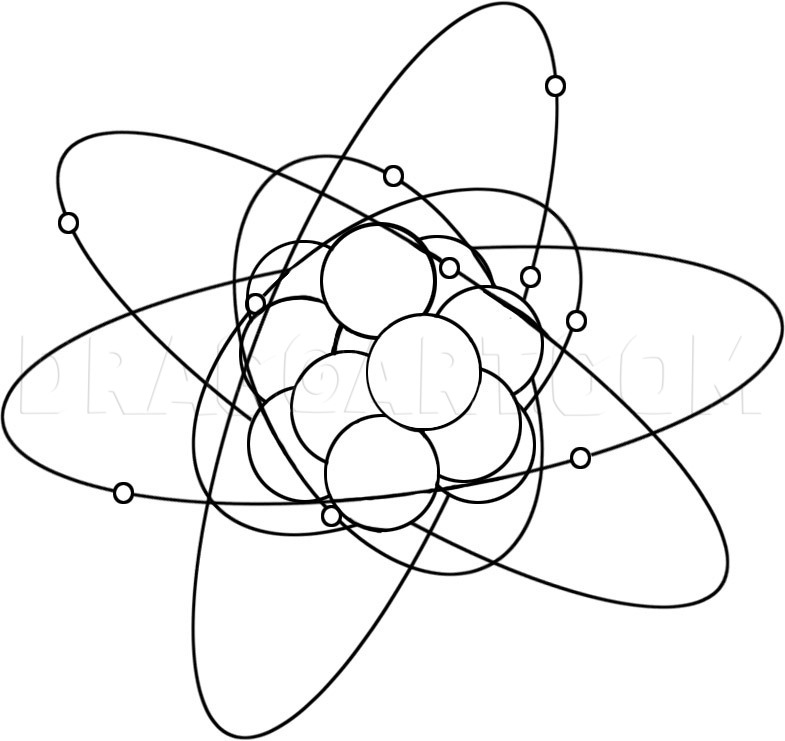
Start with a large circle for the center of the atom. Next draw a series of vertical lines that go through the center of the atom. These lines are going to be the cloud for the electrons.
2
You will now start drawing out the circles for the protons, and the neutrons that are in the center. Next draw out the tiny circle particles for the electrons and then move to the next step.
3
You will draw an oval like ring that goes around the center as seen here and this is going to be the cloud that is formed by the atoms electrons.
4
Draw another oblong oval circle and this will be done in the next step as well until there are three clouds in all.
6
You will now draw out the center clouds that surround the core of the atom and then start erasing the guidelines that you drew in step one to clean up your drawing.
Comments 0
Details
May 7, 2009
Description: Science and physics is a subject that holds the key to many answers of many questions about our world and everything in it. My brother did a homework assignment on the atom and he was supposed to draw a picture of what an atom looked like. He didn't know how to draw an atom step by step, so I had to help him in that area. Now because there was no tools for him to turn to on the internet, I decided to do a lesson on how to draw an atom. The atom is a piece of matter and matter is anything that can be physically touched by anyone or anything. Now for those of you that already know what the make up of an atom is, then I guess you can skip this part and move onto the drawing lesson. Or you can choose to read along and see if there is something new you didn't know about the atom. And for those of you that are doing a report on this subject for your science teacher, then maybe you want to continue on with the read. Everything in the universe is made of matter, which means the entire universe is all made of atoms (everything except energy that is). If you were to look at an atom itself, you would see that it is made up of three small particles which are called “subatomic particles”. These subatomic particles are; protons, neutrons, and electrons. Now, it is the protons and neutrons that make up the nucleus which is the center of the atom. Around the atom the electrons move around and above the nucleus which forms a small cloud like effect. What is the difference between electrons and protons you ask? Well, electrons hold a negative charge where as the protons carry a positive charge. When an atom is in a neutral state, the amount of protons and electrons are equal to each other and so would the neutrons in most cases, but that doesn't mean always. For instance, a magnesium atom would have; twelve protons, twelve neutrons, and twelve electrons. The protons and neutrons stay in the center and the electrons drift away from the center forming a ring like cloud. Facts about the atom is a very interesting subject to study. One of the famous scientists of the world loved it so much, he formed a formula that created the atomic bomb, and his name is Albert Einstein. I hope you guys like this lesson on how to draw an atom step by step. The tutorial is very simple so I know you will have no problems figuring out what to do next. I have to go now and draw out some new lessons for you all so peace out, and catch you guys later.